contact
Yulvonnda Brown
Office Manager
F 410-706-0865
Dr. Ro Projects
Receptor-channel and channel-channel interactions in trigeminal sensory neurons
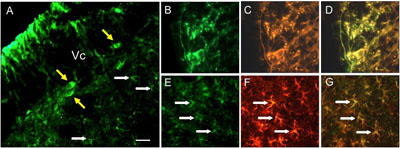
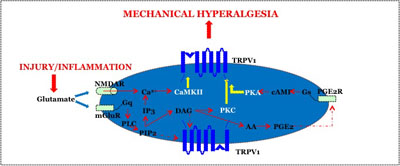
This project investigates peripheral neural mechanisms that underlie the development of mechanical hyperalgesia; a prominent clinical feature associated with persistent muscle pain conditions. We have previously shown that peripherally localized NMDA receptor (NMDAR) and metabotropic glutamate receptor 5 (mGluR5) are important components in evoking acute muscle nociception as well as mechanical hyperalgesia. Several members of the transient receptor potential (TRP) family, particularly TRPV1 and TRPA1, also play an essential role in the development of mechanical hypersensitivity under various pain conditions. Since activation of peripheral glutamate receptors invokes various intracellular signaling cascades leading to nociceptor sensitization, and both TRPV1 and TRPA1 are suggested to function as ‘inflammatory signal integrators’, we propose that NMDAR/mGluR5 and TRPV1/TRPA1 are functionally coupled and that activation of NMDAR/mGluR5 leads to TRPV1/TRPA1-dependent nociception and mechanical hyperalgesia via multiple intracellular signaling pathways. We evaluate functional interactions between NMDAR/mGluR5 and TRPV1/TRPA1 with behavioral pharmacology and in vivo RNAi studies, and morphological interactions with immunohistochemical and biochemical experiments in trigeminal ganglia (TG) muscle afferents. We also investigate specific intracellular signaling pathways underlying NMDAR/mGluR5 and TRPV1 interactions in TG, and intracellular mechanisms underlying NMDAR/mGluR5 and TRPA1 interactions. The integrated studies proposed here will provide comprehensive information on novel mechanisms of peripherally mediated mechanical hyperalgesia, and have immediate translational implications in a relatively understudied area of clinical muscle pain conditions, such as temporomandibular disorders.
Sex differences in cannabinoid and opioid recepor mechanisms
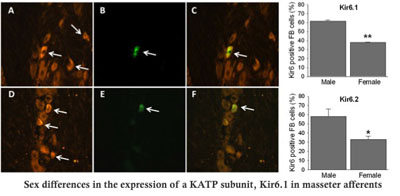
This project investigates the factors that contribute to sex differences in molecular, cellular, and function properties in peripheral opioid receptor (POR) and cannabinoid receptor (CBR) systems. We focus on examining novel mechanisms by which sex differences in peripherally applied opioid/cannabinoid reeptor agonists are expressed in the context of inflammatory muscle pain conditions. The central hypotheses of this project are that sex differences in POR/CBR mechanisms are expressed at multiple levels in primary afferent signaling process and injury or inflammation differentially impacts POR/CBR signaling between the sexes. Studies will determine whether there are sex differences in (1) expression levels of the three subtypes of ORs, μ, δ, κ ORs, and CB1R; (2) sub-cellular localizations of the three OR subtypes and CB1R; (3) the expression of major downstream targets, G-protein coupled and ATP dependent inward rectifying potassium channels (GIRK and KATP), under normal and inflammatory conditions. (4) We are also examining how various inflammatory cytokines and sex steroid hormones modulate POR/CBR expression. Each of these studies will be accompanied by behavioral tests to assess functional relevance of molecular and cellular changes. These studies bear high clinical significance since the pain and management involving masticatory muscles and TMJ, such as in TMJMD, are sexually dimorphic, and since there is increasing clinical as well as pre-clinical evidence that indicate PORs as potential therapeutic targets for treating various types of chronic pain conditions. Understanding mechanic bases for sex-differences in POR/CB1R function will help develop sex-specific management strategies for persistent types of orofacial muscle pain.
The role of three nitric oxide synthases (NOS) in orofacial pain
In this project, we investigate the functional role of centrally released nitric oxide (NO) in the pathogenesis of orofacial muscle pain. NO, which has been implicated in the development of neuronal hyperexcitability, has not been systematically studied in the trigeminal system, especially in the context of inflammatory muscle pain condition. In light of recent studies that underscore the significance of glia and neuron interaction in the development of chronic pain conditions, elucidating relative contributions and temporal profiles of NO synthesized and released from both neurons and non-neuronal cells in the trigeminal sensory nuclear complex (TSNC) is of considerable clinical importance. The central hypotheses of this proposal are that that acute and chronic masseter inflammation differentially regulate nitric oxide synthase (NOS) from neuronal and non-neuronal cells (i.e., nNOS, iNOS, and eNOS), that excess NO release leads to muscle tissue hypersensitivity via PKG dependent mechanisms, and that blockade of NO-sGC-cGMP pathway results in attenuation of orofacial muscle pain and hyperalgesia. A series of experiments are proposed to test these hypotheses. Initially, Western blot and immunohischemical studies will assess the extent and localization of the three NOS enzymes in the TSNC before and after experimental induction of acute or chronic myositis. The functional role of NO will be then tested by pharmacological manipulations of each NOS enzyme under acute and chronic inflammatory conditions with the behavioral models specifically developed for assessing craniofacial muscle tissue sensitivity. Finally, we will attempt to block the NO mediated muscle pain and hyperalgesia by silencing the gene that encodes soluble guanylate cyclase (sGC), a physiological receptor for NO, in the TSNC. The in vivo delivery of siRNA offers a powerful technique of selectively inhibiting one or multiple genes in intact animals.