contact
Shereece Singleton
Office Manager
F 410-706-0865
Vaccine Development Against Pathogens Causing Chronic Infections in Wounded Warriors
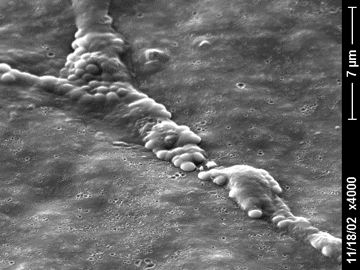 This DOD funded project seeks to find vaccine candidates against pathogens that cause chronic biofilm infections in returning soldiers including Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
This DOD funded project seeks to find vaccine candidates against pathogens that cause chronic biofilm infections in returning soldiers including Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Background: The recurrences of infections in traumatic musculoskeletal injuries are the result of microbial biofilm formation on the implant. This microbial community enables the persistence of the microbial species that cause the vast majority of these battlefield musculoskeletal infections (Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae). A biofilm may be defined as a microbe-derived sessile community characterized by cells that are attached to a substratum, interface, or each other, are embedded in a matrix of extracellular polymeric substance, and exhibit an altered phenotype with respect to growth, gene expression, and protein production. Biofilm growth enables resistance to removal strategies, most notably antimicrobials and host response.
Hypothesis: We hypothesize that the production levels of specific proteins are up-regulated in microbial biofilms and immunization with these proteins may elicit a protective response that will prevent the development of biofilm infection in an established rabbit osteomyelitis model and mouse model of tibial implant infection.
Specific Aims: (1) To identify A. baumannii, P. aeruginosa, and K. pneumoniae proteins with up-regulated production in a biofilm mode of growth by two-dimensional gel electrophoresis and mass spectrometry; (2) To identify those biofilm proteins found in Specific Aim 1 that are recognized by the immune system in a rabbit war wound osteomyelitis model of infection; (3) To demonstrate the protective efficacy of the biofilm proteins identified in Specific Aims 1 and 2 in a war wound rabbit osteomyelitis model of infection; (4) To design and develop a multicomponent vaccine against the three microbial pathogens, A. baumannii, P. aeruginosa, and K. pneumoniae; and (5) To demonstrate the preventive efficacy of the vaccine candidate in a rabbit war wound model of infection and mouse model of tibial implant infection.
Study Design: We will evaluate selected up-regulated proteins for their ability to be recognized by the host immune system during an in vivo biofilm infection, and second, for their protective efficacy in preventing osteomyelitis infection in a rabbit model. Third, we will develop a multicomponent vaccine against the three microbial pathogens, A. baumannii, P. aeruginosa, and K. pneumoniae. Fourth, we will test the vaccines for their efficacy in our war wound rabbit osteomyelitis model and mouse model of tibial implant infection. In addition, this research will also contribute to a more complete understanding of the bacterial factors involved in microbial biofilm formation and maturation
Relevance: This understanding will enable the creation of novel materials, surfaces, and/or disinfection strategies that resist or eliminate bacterial fouling and biofilm formation, thus providing a new weapon in the fight against persistent musculoskeletal infections resulting from trauma or on medical implants.
