contact
Nancy Kamphaus
Administrative Assistant
Do not use this number or leave any messages at this exchange for Oral Medicine Consultants or Oral Pathology Consultants
Professor Meenakshi Chellaiah, PhD
My lab research focuses on bone remodeling due to modulation in osteoclasts and osteoblasts in response to various treatments and cancer metastasis to bone. I have provided a summary of the ongoing projects in the lab.
Research Interests
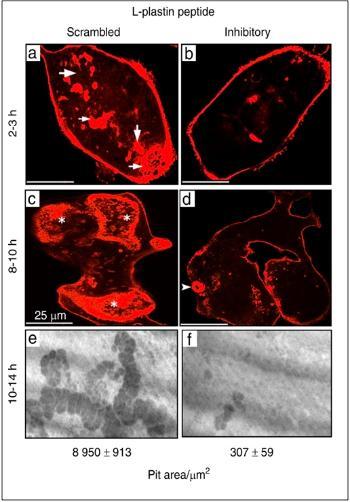
- Project 1: My laboratory is the first to document L-plastin's role (LPL) in osteoclast sealing ring formation and bone resorption in the osteoclast project. L-plastin (LPL) was identified as a potential regulator of the actin-bundling process involved in forming nascent sealing zones (NSZs), which are precursor zones for mature sealing rings. Genetic deletion of LPL in mice demonstrated decreased eroded perimeters and increased trabecular bone density. TAT-fused cell-penetrating small molecular weight LPL peptide (TAT- MARGSVSDEE, denoted as an inhibitory LPL peptide) attenuated the formation of NSZs and sealing rings and impaired bone resorption in vitro in osteoclasts (Figure below).
- Subsequently, we injected aging C57BL/6 female mice (36 weeks old) subcutaneously with the inhibitory and scrambled peptides of LPL for 14 weeks. Micro-CT and histomorphometry analyses demonstrated an increase in trabecular bone density of femoral and tibial bones with no change in cortical thickness in mice injected with the inhibitory LPL peptide. The LPL peptide approach in bone loss is novel. We will examine the impacts of the LPL inhibitory peptide on other models that display bone loss due to osteoclast activation. To improve stability and sustain release, we are using nanotechnology, which was shown to have more advantages in delivering proteins and peptides.
- This inhibitory LPL peptide has been patented, and it has been publicly available at http://www.uspto.gov/patft/. The patent application number and date are provided by the United States Patent and Trademark Office (United States Department of Commerce).
- Project 2: Recently, we developed another project to determine C-phycocyanin (C-PC) effect on LPS regulated osteoclastogenesis. C-PC strongly inhibited the early stage of osteoclast differentiation, thus significantly suppressing RANKL- TNF-alpha- and LPS- mediated osteoclastogenesis. Results from our studies highlighted a possible osteoclastogenesis process during bacterial-mediated osteolytic infections. Therefore, targeting LPS-induced osteoclastogenesis could be a promising therapeutic strategy to inhibit inflammatory bone loss in diseases such as periodontitis, rheumatoid arthritis, etc. Future investigations will use proper animal model systems to determine C-PC's effect on bone loss in vivo.
- Project 3: We are using dental stem cells to generate osteoblast-like cells. Our focus here is how these stem cells can be utilized with osteoinductive materials and stimulatory factors (e.g., Methylsulfonylmethane (MSM)) to induce bone formation. We found that MSM can increase the osteogenesis process and regulate the matrix's mineralization via transglutaminase enzyme in SHED cells (stem cells). We continue to investigate the effect of MSM in an aging mice model system to validate its impact on bone formation in vivo.
- Project 4: Prostate cancer (PCa) is the second leading cause of cancer-related death in men in the United States, partially due to metastatic spread to secondary sites in the bone, brain, lymph nodes, and visceral organs. Here, we examine the mechanisms that can facilitate PCa progression/metastasis. We identified the critical role of the intracellular domain of CD44 in metastasis relevant events, including migration and tumorigenesis. For the first time in PCa, we showed the consequence of CD44 intracellular domain cleavage on tumorigenesis and the sequence-specific interaction with RUNX2, a transcriptional factor involved in the transcription of metastasis-related genes. Our results allude to CD44-ICD as a novel therapeutic target in cancer cells that express CD44. Future studies will focus on CD44-ICD signaling, interactions, and tumorigenesis.
Publications pertinent to the projects mentioned above are provided below.
 |
|
Osteoclasts were stained for actin with rhodamine-phalloidin, and confocal images of osteoclasts are shown (a–d). Osteoclasts plated on dentine matrix incubated with scrambled (a and c) LPL peptide in the presence of TNF-α for 2-3h demonstrate nascent sealing zones (NSZs, a; indicated by arrows) and for 8-10h demonstrate mature sealing rings (SRs, c; indicated by asterisks). Cells incubated with inhibitory peptide (b and d) fail to demonstrate either NSZs (b) or SRs (d). An arrowhead points to a small sealing ring in (d). Scale Bar-25 µm. e and f Dentine resorption assay in vitro: Osteoclasts plated on dentine matrix were incubated with scrambled (e) or inhibitory (f) LPL peptide in the presence of TNF-α for 10–14 h. Resorption pits were photographed under a 40X objective of phase-contrast microscopy (magnification is ×400). Resorbed areas of approximately ~25–30 pits were quantified from three slices per treatment/experiment (n = 3) and averaged over two different experiments. Statistical analysis of the pit area was performed by Student’s test and provided at the bottom. ***P < 0.001 vs. scrambled peptide transduced osteoclasts (mean ± SEM). [Bone Res 9, 22 (2021] |
Peer-Reviewed Publications Since 2018
- Aljohani H, Senbanjo LT, Stains J, and Chellaiah MA. Methylsulfonylmethane increases the alveolar bone density of mandibles in aging female mice. Front. Physiol. 12:708905. doi: 10.3389/fphys.2021.Oct.4; 708905
- Chellaiah MA. L-Plastin Phosphorylation: Possible Regulation by a TNFR1 Signaling Cascade in Osteoclasts. Cells 2021 Sept 9; 10(9), 2432; https://doi.org/10.3390/cells10092432
- Majumdar S, Senbanjo LT, AlJohani H, Chellaiah MA. L-plastin regulates invasion and possibly not the migration of prostate cancer (PC3) cells. Journal of Cancer Research and Therapeutic Oncology 2021 9: 1-17.
- Aljohani H, Stains JP., Majumdar S, Srinivasan D, Senbanjo L, Chellaiah MA. Peptidomimetic inhibitor of L-plastin reduces osteoclastic bone resorption in aging female mice. Bone Res 2021 Apr 9; 9: 22 (2021). https://doi.org/10.1038/s41413-020-00135-9.
- AlQranei MS, Senbanjo LT, Aljohani H, Hamza T, Chellaiah MA. Lipopolysaccharide- TLR-4 Axis regulates osteoclastogenesis independent of RANKL/RANK signaling. BMC Immunol. 2021 Mar 25;22(1):23. doi: 10.1186/s12865-021-00409-9. PubMed PMID: 33765924; PubMed Central PMCID: PMC7995782.
- Senbanjo LT, AlJohani H, AlQranei M, Majumdar S, Ma T, Chellaiah MA. Identification of sequence-specific interactions of the CD44-intracellular domain with RUNX2 in the transcription of matrix metalloprotease-9 in human prostate cancer cells. Cancer Drug Resist. 2020 3rd Quarter;3(3):586-602. doi: 10.20517/cdr.2020.21. Epub 2020 Aug 21. PubMed PMID: 33062960; PubMed Central PMCID: PMC7556329.
- Chellaiah MA. Regulation of Bone Resorption by Osteoclasts-an Overview. Medical Research Archives. 2020 August; 8(9):1-10.
- Chellaiah MA. Osteoclast Cytoskeleton, Podosome, Motility, Attachment, and Signaling by Receptors. Encyclopedia of Bone Biology: Zaidi, M (ed.) Oxford: Academic Press. 2020 July; 1:236-250.
- AlQranei MS, Chellaiah MA. Osteoclastogenesis in periodontal diseases: Possible mediators and mechanisms. J Oral Biosci. 2020 Jun;62(2):123-130. doi: 10.1016/j.job.2020.02.002. Epub 2020 Feb 17. Review. PubMed PMID: 32081710; PubMed Central PMCID: PMC7329621.
- AlQranei MS, Aljohani H, Majumdar S, Senbanjo LT, Chellaiah MA. C-phycocyanin attenuates RANKL-induced osteoclastogenesis and bone resorption in vitro through inhibiting ROS levels, NFATc1 and NF-κB activation. Sci Rep. 2020 Feb 13;10(1):2513. doi: 10.1038/s41598-020-59363-y. PubMed PMID: 32054921; PubMed Central PMCID: PMC7018981.
- Chellaiah MA, Moorer MC, Majumdar S, Aljohani H, Morley SC, Yingling V, Stains JP. L-Plastin deficiency produces increased trabecular bone due to attenuation of sealing ring formation and osteoclast dysfunction. Bone Res. 2020;8:3. doi: 10.1038/s41413-019-0079-2. eCollection 2020. PubMed PMID: 31993243; PubMed Central PMCID: PMC6976634.
- Aljohani H, Senbanjo LT, Chellaiah MA. Methylsulfonylmethane increases osteogenesis and regulates the mineralization of the matrix by transglutaminase 2 in SHED cells. PLoS One. 2019;14(12): e0225598. doi: 10.1371/journal.pone.0225598. eCollection 2019. PubMed PMID: 31805069; PubMed Central PMCID: PMC6894810.
- Senbanjo LT, Chellaiah MA. CD44 cleavage product CD44-intracellular domain regulates gene transcription and tumorigenesis. Cancer Studies and Therapeutics. 2019 September; 4(4):1-2.
- Senbanjo LT, AlJohani H, Majumdar S, Chellaiah MA. Characterization of CD44 intracellular domain interaction with RUNX2 in PC3 human prostate cancer cells. Cell Commun Signal. 2019 Jul 22;17(1):80. doi: 10.1186/s12964-019-0395-6. PubMed PMID: 31331331; PubMed Central PMCID: PMC6647163.
- Majumdar S, Wadajkar AS, Aljohani H, Reynolds MA, Kim AJ, Chellaiah MA. Engineering of L-plastin Peptide-Loaded Biodegradable Nanoparticles for Sustained Delivery and Suppression of Osteoclast Function In Vitro. Int J Cell Biol. 2019;2019:6943986. doi: 10.1155/2019/6943986. eCollection 2019. PubMed PMID: 31191656; PubMed Central PMCID: PMC6525930.
- Homayounfar N, Khan MM, Ji Y, Khoury ZH, Oates TW, Goodlett DR, Chellaiah M, Masri R. The effect of embryonic origin on the osteoinductive potential of bone allografts. J Prosthet Dent. 2019 Apr;121(4):651-658. doi: 10.1016/j.prosdent.2018.09.003. Epub 2018 Dec 28. PubMed PMID: 30598313.
- Chellaiah MA, Ma T, Majumdar S. L-plastin phosphorylation regulates the early phase of sealing ring formation by an actin-bundling process in mouse osteoclasts. Exp Cell Res. 2018 Nov 1;372(1):73-82. doi: 10.1016/j.yexcr.2018.09.014. Epub 2018 Sep 21. PubMed PMID: 30244178; PubMed Central PMCID: PMC6181588.
- Chellaiah MA, Majumdar S, Aljohani H. Peptidomimetic inhibitors of L-plastin reduce the resorptive activity of osteoclast but not the bone-forming activity of osteoblasts in vitro. PLoS One. 2018;13(9):e0204209. doi: 10.1371/journal.pone.0204209. eCollection 2018. PubMed PMID: 30248139; PubMed Central PMCID: PMC6152981.
- Srinivasan D, Senbanjo L, Majumdar S, Franklin RB, Chellaiah MA. Androgen receptor expression reduces stemness characteristics of prostate cancer cells (PC3) by repression of CD44 and SOX2. J Cell Biochem. 2018 Sep 11; 120(2):2413-2428. doi: 10.1002/jcb.27573. [Epub ahead of print] PubMed PMID: 30206982; PubMed Central PMCID: PMC6411465.
Lab Personnel (Present and Past)
Current Graduate Student
Marjan Sharifi, DDS (since 2019)
Past members since 2000
Post-doctoral fellows (2000 to 2020)
Rajat Biswas, PhD
Venkatasababa Samanna, PhD
Tao Ma, PhD
Aditi Gupta, PhD
Sunipa Majumdar, PhD
Linda Senbanjo, PhD
Research Fellow (2016-2018)
Deepa Srinivasan, MS
Graduate students (2004-2020)
(graduated years in parenthesis)
Bhavik Desai (2008)
Brian Robertson (2010)
Linda Senbanjo (2019)
Hanan Aljohani (2020)
Mohammed AlQranei (2020)
Research Assistants
David Yuen (2000-2002)
Kavitha Sadashivaiah (2009)
Exchange Visitors
Ganglao Gao, a physician from China (2013)
Lei Lei (China; 2012)
Han Ji Young (Korea; 2012)
Hongzhi Zhou (Physician and Professor from China Came on Sabbatical; 2011)
Wei Cao (Ph.D. candidate from Dr. Wantao Chen laboratory; China; 2010)
Dr. Chellaiah Lab also supported laboratory rotations of several Master's and Ph.D. students and residents' projects at Dental School.
Education and Training
| 1980-1985 | Ph.D., Biochemistry | Madurai Kamaraj University, India |
| 1975-1977 | M.Sc., Zoology | Madurai Kamaraj University, India |
| 1972-1975 | B.Sc., Zoology | Madurai Kamaraj University, India |
| 1997-1999 | Research Instructor, Department of Medicine; Washington University School of Medicine, St. Louis |
| 1992-1997 | Research Associate, Department of Medicine; Jewish Hospital, St. Louis, Missouri |
| 1990-1992 | Research Associate, Department of Pathology; Jewish Hospital of St. Louis, St. Louis, Missouri |
| 1987-1990 | Postdoctoral Fellow, Department of Biochemistry & Molecular Biology; St. Louis University, St. Louis, Missouri |
| 1986-1987 | Research Associate, Department of Microbiology; Madurai Kamaraj University, Madurai, India |
| 1977-1979 | Research Assistant, Department of Biochemistry; Madurai Kamaraj University, Madurai, India |
Employment History
| August 2014‑Present | Professor, Department of ODS; SOD, University of Maryland, Baltimore |
| Aug 2007-July 2014 | Associate Professor, Department of ODS; SOD, University of Maryland, Baltimore |
| 2001-present | Adjunct Assistant Professor, Molecular and Cell Biology Graduate program; University of Maryland, Baltimore |
| July 2000-2007 | Assistant Professor, Department of BMS; SOD, University of Maryland, Baltimore |
| 1999-June 2000 | Research Assistant Professor, Department of Medicine; Washington University School of Medicine, St. Louis, Missouri |
Honors and Awards
| 2010-2013 | NIH-NIAMS Bridge Award ($337 500) |
| 2009-2010 | American Society for Bone and Mineral Research Award ($50,000) |
| 2001-2002 | DRIF: University of Maryland, Dental School $4500 |
| 2002-2003 | DRIF: University of Maryland, Dental School $4500 |
| 1999-2000 | Barnes-Jewish Research Foundation, St. Louis, MO $50,000 |
| 1996-1997 | Barnes-Jewish Research Foundation, St. Louis, MO $50,000 |
| 1991-1992 | NIADDK training grant award |
| 1989-1990 | NRS training grant award |
| 1980-1985 | Council of Scientific and Industrial Research Award for Ph.D. program |
| 1979 | University IV Rank Holder in MSc (India) |
| 1977-1979 | Awarded National Merit Scholarship for Master program (MSc) in India |
| 1977 | University II Rank Holder in bachelor's degree (BSc) for which National Merit Scholarship was awarded (India) |
Grants
Completed Grants
- Proposal Title: "L-Plastin: A Novel Target for Intervention in the Treatment of Osteoporosis." Principal Investigator. NIH/NIAMS. NIH R01 AR066044-01 (09/15/2014-08/30/2019). No Cost Extension until 2021
- Competitively renewed twice R01 Grants (08-01-1999 to 06- 30-2013): Proposal Title: "Regulation of signaling in osteoclast bone resorption" NIH/NIAMS. Principal Investigator. 2 R01 AR-46292-9A1 was competitively renewed twice during this period.
- Bridge Award for one year: Principle Investigator. Agency: American Society for Bone and Mineral Research
- F30 Student Grant (Awarded for two years): Role-Mentor. Awarded to student Brian Robertson (graduate student in DDS/Ph.D. program). Title: The role of osteopontin in cancer progression and metastasis Period: 06-01-07 to 06-31-09. NIH-NIDCR. Type: 1 F30 DE018308-01

